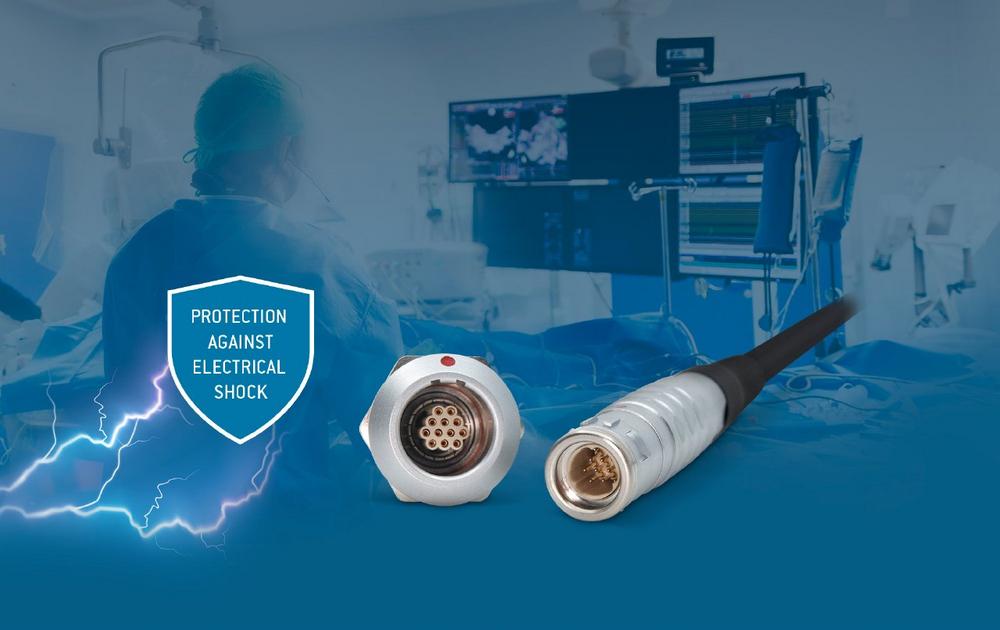
The portfolio now includes additional inserts that have been specially designed to fulfil the user and patient protection levels required by IEC 60601-1 (2 MOOP/ 2 MOPP). These connectors now become the solution of choice when searching for a circular connector with a metal housing for medical applications.
The approval procedures for medical electrical equipment and systems have become even more complex than they already were prior to the release of the latest version of the IEC 60601-1. If ODU is involved in the design-in process from the start then not only is the risk management process simplified, but also the cost of product development for such manufacturers can be reduced to a minimum. At the end of the day, the necessary time for approval procedures can be substantially shortened if components from suppliers are already fully compliant with the IEC 60601-1. This is the case with this standard portfolio extension from ODU.
The main advantages of choosing a connector with a metal housing for fulfilment of the requirements defined under IEC 60601-1 (2 MOOP/ 2 MOPP) are the advanced electromagnetic shielding capabilities and the mechanical strength of the connector. These features ensure safe operation and the best possible signal quality in the most demanding of medical environments.
The new inserts are available in 3 sizes and offer a choice of contacts as follows (further contact variants available on request):
- Size 1: 7 crimp contacts, ∅ 0,7 mm, Nominal Voltage 36V AC or DC
- Size 2: 12 crimp contacts, ∅ 0,7 mm, Nominal Voltage 48V AC or DC
- Size 3: 16 crimp contacts, ∅ 0,7 mm, Nominal Voltage 48V AC or DC
The inserts are suitable for deployment in the ODU MINI-SNAP® L- & K-Series of connectors. Reverse gendering options are also available. The above-mentioned voltage ratings are valid for pollution grade 2 (IEC 60601-1). The nominal current rating is specified with 7A.
Customer specific cable assemblies with or without silicone overmolding are also available upon request.
These products, together with the successful ODU MEDI-SNAP® portfolio, further strengthen ODU’s position in serving the medical electrical equipment industry with IEC 60601-1 compliant solutions.
Further IEC 60601-1 compliant product series are the ODU MEDI-SNAP® and ODU MINI-MED®. See the following link for a whitepaper on IEC60601-1: https://www.odu-connectors.com/downloads/whitepapers/ and for the products https://www.odu-connectors.com/products/circular-connectors/odu-mini-snap/pin-and-groove-coding/
The ODU Group is one of the world’s leading suppliers of connector systems, employing 2,300 people around the world. In addition to its company headquarters in Mühldorf am Inn (Germany), ODU also has an international distribution network and production sites in Sibiu/Romania, Shanghai/China and Tijuana/Mexico. ODU combines all relevant areas of expertise and key technologies including design and development, machine tooling and special machine construction, injection, stamping, turning, surface technology, assembly and cable assembly. The ODU Group sells its products globally through its sales offices in China, Denmark, England, France, Germany, Hong Kong, Italy, Japan, Korea, Romania, Sweden and the US, as well as through numerous international sales partners. ODU connectors ensure a reliable transmission of power, signals, data and media for a variety of demanding applications including medical technology, military, communications and security, automotive, industrial electronics, and testing & measurement.
ODU GmbH & Co. KG
Pregelstraße 11
84453 Mühldorf a. Inn
Telefon: +49 (8631) 6156-0
http://odu-connectors.com/de/
Head of Corporate Marketing & Communications
Telefon: +49 (8631) 6156-1691
Fax: +49 (8631) 6156-4691
E-Mail: Nicol.Schindlbeck@odu.de
![]()