However, the research situation and the associated information of the population is rather diffuse. Press releases outdo each other with the issuing of hastily published research results, and the uncertainty of patients is growing. On the one hand, reports state that patients who have recovered from an infection are protected against reinfection. On the other hand, the protection is said to be based on the strength of the previous infection, and following that, a third party promptly reports reinfections. In this jumble of reports, investigations of the late consequences of SARS-CoV-2 infections are almost lost.
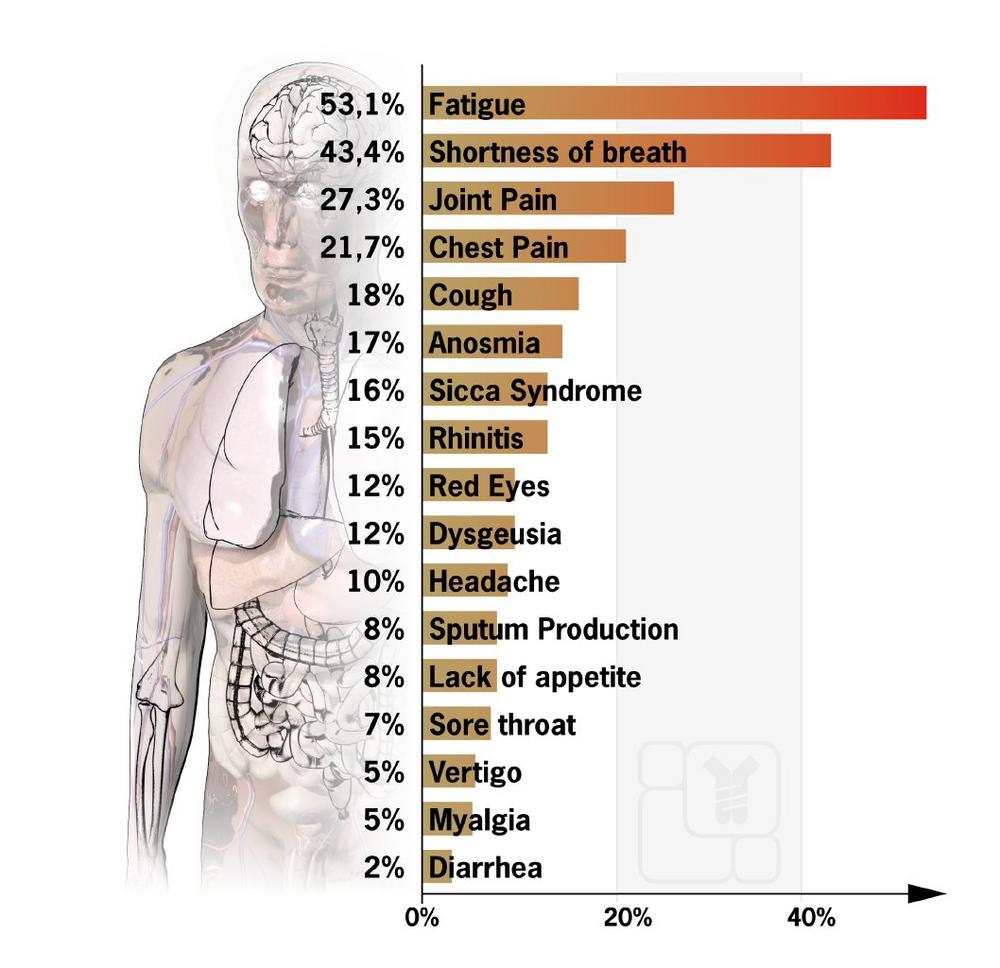
Such late effects can vary from patient to patient, and many factors play a role. According to a recent study (Carfi A. et al. 2020) of 143 patients treated in Italian hospitals, 87 percent of them still suffered from at least one symptom 60 days after the disease’s onset. A high proportion of people still reported fatigue (53.1%), shortness of breath (43.4%), joint pain (27.3%), and chest pain (21.7%). On average, only slightly more than one in ten patients reported being free of any symptoms.
While the frequently cited symptoms mainly sound like the late effects of pneumonia, which in most cases is the most severe of the COVID-19 diseases, an infection with the SARS-CoV-2 virus can also damage the heart muscle, intestine, kidneys, vascular lining, and the nervous system.
In individual cases, Guillain-Barré syndrome also occurs as an inflammatory disease of the nerves. The Syndrome can lead to paralysis of the legs, arms, trunk, and respiratory muscles. According to the German Society of Neurology, these problems occur not only in patients who belong to the risk group but also in those who were perfectly healthy before their infection. The research group around Harriet Kemp from Imperial College London (Kemp H. et al. 2020) also reports patients who complain of partially diffuse chronic pain after a completed SARS-CoV-2 infection.
The AESKU® product portfolio offers a wide range of possibilities to investigate and monitor such late effects and exclude a possible autoimmune disease freshly established after a severe SARS-CoV-2 infection.
For example immunoblot tests of the AESKUBLOTS® range on Vasculitis, Myositis, Gastroenterology and Rheumatology, or ELISA-tests from the AESKULISA® product lines Hemostasis, Gastroenterology, Vasculitis, Thrombosis and Rheumatology
But furthermore, the current threat of SARS-CoV-2 must not be lost out of sight. For the exclusion of an acute or recent infection, our quantitative AESKULISAs SARS-CoV-2 IgA, IgM, and IgG are recommended.
Among others, our AESKUBLOTS®
4001 ANA-17 Pro
4002 Vasculitis Pro
4003 Myositis Pro
4005 Gastro Pro
4008 ANA-17 Comp
and our AESKULISA®:
Hemostasis
3901 Protein C
3902 Protein S
Gastroenterology
3500 Gliadin Check
3509 Crohn‘s Check
3510 CeliCheck New Generation
3515 DGP Check
Vaskulitis
3301 ANCA-Pro
3302 PR3 sensitive
3303 MPO
3305 Elastase
3323 Vasculitis-Screen
Thrombosis
3202 Cardiolipin-Check
3211 Prothrombin-Check
3219 Phospholipid-Screen-A
3225 Thrombin-Check
3291 Hit II-Check
3324 Phospholipid-Screen GM
Rheumatology
3100 ANA-8S
3101 ANA-8Pro
3164 α-Fodrin-Check
3168 MMP-3
3190 SpA-Detect
shall be mentioned here.
Wendelsheim based AESKU.GROUP, composed of AESKU.DIAGNOSTICS, AESKU.SYSTEMS, AESKU.KIPP INSTITUTE, AESKU.THERAPY, AESKU.DST & AESKU.BION, offers the most comprehensive product portfolio in the field of autoimmune diagnostic – worldwide. AESKU.GROUP is proud to provide its state of the art Products (using ELISA (AESKULISA®), BLOT (AESKUBLOTS®) or Immunofluorescence (AESLUSLIDES®) methodology), covering the wide area of autoimmunity, infectious serology, allergy and food intolerance.
AESKU.GROUP, as a company, is devoted to providing outstanding diagnostic, software and systems solutions, scaled to the modern Labs‘ needs.
Within a short time, AESKU.DIAGNOSTICS becomes a recognized name in autoimmunity diagnostics and still offers the most comprehensive product portfolio worldwide. Acquiring DST in 2017 – a specialized company in allergy and food intolerance, with over 600 in-house developed and produced allergens – AESKU.GROUP added the expertise in allergy and food intolerance to its already wide ranged set of talents. AESKU.BION specializes in manufacturing high quality IFA products for clinical laboratories. The company is FDA-registered, and ISO 13485:2016 certified. They are experts in IFA for Immunology, virology, and microbiology.
The high quality and innovativeness of AESKU® products has its foundation on the in-house research, development and production of all assays and lab systems. This ensures the best possible quality and consistency to the customer.
AESKU.GROUP employs over 300 dedicated people of more than 20 different nationalities, offering over 320 different products in more than 90 countries around the worldwide. AESKU.GROUP has local branches in Germany, the US, Italy, UK, Singapore, Columbia and China.
AESKU.GROUP GmbH
Mikroforum Ring 2
55234 Wendelsheim
Telefon: +49 (6734) 9622-0
Telefax: +49 (6734) 9622-2222
http://www.aesku.com
Marketing Coordinator
Telefon: +49 (6734) 9622-0
Fax: +49 (6734) 9622-2222
E-Mail: koch@aesku.com
Telefon: +49673496220
E-Mail: info@aesku.com
![]()